Abstract
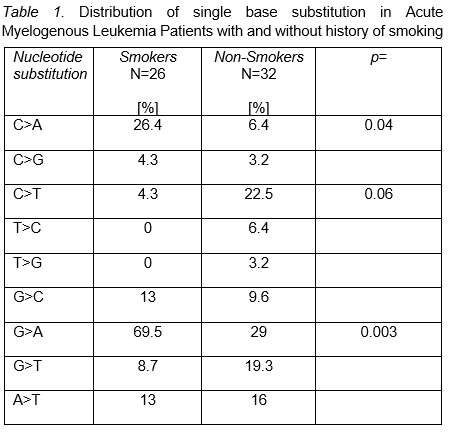
Background: Endogenous and exogenous processes are active in leukemia initiation. Single-base substitution (SBS) data permits aggregation into mutational signatures [MS] with potential for clinical application. Acetaldehyde and Benzo [a] pyrene (BaP) are tobacco mutagens that "drive" signature SBS4 [Catalog of Somatic Mutations in Cancer (COSMIC) https://cancer.sanger.ac.uk/signatures/sbs/] in lung cancer characterized by C>A transversion. This specific MS is associated with favorable response to immune-checkpoint therapy (ICT). Previous epidemiologic data correlate smoking with cancer including leukemia. Tobacco mutagens are ubiquitously distributed in organs once inhaled. However, how mutagenicity develops in hemopoietic stem cell/progenitor is unknown. In this study, our primary objective was to investigate the incidence of MS among patients (pt) diagnosed with Acute Myelogenous Leukemia (AML) with smoking exposure. Methods: After IRB approval, we performed retrospective analysis using the BCM AML database. Data from 58 AML pt was available. For all analysis, current and past smoking were aggregated into "positive exposure". SBSs (C>A, C>G, C>T, T>C, T>G, G>C, G>A, GT and AG) in smokers and never smoker were annotated from ELN 2017 predefined subgroups [i.e. CEBPA, NPM1, P53, ASLX1, RUNX1 and FLT3ITD] and all additional mutations in individual pt. We used descriptive statistics to detect differential clinical predictors for smoking induced MS. Chi-square was used to determine association between SBSs and smoking history. Stepwise logistic regression allowed identification of independent MS that correlated with smoking. Results: Median age for 58 AML pt was 65.5 years [y] (range, 22-89) and 58.3% were male. Smokers were 26/58 (44.8%). Whites, African Americans, Hispanics and Asians comprised 35/60 (58.3%), 7/60 (7.1%), 16/60 (26.6%) and 2/60 (2.3%), respectively. 112 myeloid mutations [91 SBSs, 16 duplications, 16 deletions, and 3 insertions] were recorded. 32/58 (55.1%) had positive smoking exposure. Previous reports suggest that C>A [COSMIC=SBS4], G>C [COSMIC= SBS2 and SBS13] and T>C [COSMIC=SBS5] retain strong smoking association with cancer. However, in addition to C>A [HR=0.10 (0.01-0.6), p=0.02], our logistic model identified G>A, HR=0.12 (0.02-0.4), p=0.002, as predictors of exposure. C>A+G>A MS was observed in 19/25 (76%) of AML pt with smoking exposure, OR=6.56 (1.8-23.9), p=0.002. By ELN-2017 defined subgroups, P53, ASXL1, RUNX1, FLT3 and NPM1 mut were detected in 11/58 (18.9%), 5/58 (8.6%), 4/58 (6.8%), 13/58 (22.4%) and 5/58 (8.6%). RAS was seen in 12/58 (20.6%), IDH 12/58 (20.6%), DNMT3A 10/58 (17.2%) and TET2 7/58 (12%). Interestingly, among smokers exhibiting or not C>A+G>A SBP substitution, P53 was identified in 3/3 (100%) v 0/3 (0%), p=0.05 and RAS in 75% v 25%, p=0.08. Conclusions: Our data suggest that C>A and G>A SBS substitutions are frequently observed in AML pt with smoking exposure. Hemopoietic stem cell/progenitors exposed to smoking products may initiate similar SBSs substitutions as those observed in tobacco induced solid tumors. Previously, lung cancer studies demonstrated that TP53 and KRAS mutations tumors exhibited high rate of C>A transversion associated with tumor-infiltrating lymphocytes (TIL) and high program-death 1 ligand (PD-L1) expression. Further similar studies are needed in adult diagnosed with P53 AML.
Mims: IDEC: Current holder of individual stocks in a privately-held company; Biogen: Current holder of individual stocks in a privately-held company; Incyte: Research Funding; Pfizer: Research Funding; AVEO: Research Funding; Celgene: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal